In vitro rooting and acclimatization of ‘Marubakaido’ apple rootstock using indole-3-acetic acid from rhizobacteria
Juliana Aparecida Souza1,3, Jean Carlos Bettoni1, Murilo Dalla Costa2, Tiago Celso Baldissera2, João Frederico Mangrich dos Passos2 and Silmar Primieri3
1The New Zealand Institute for Plant and Food Research Limited, Palmerston North, Manawatu, New Zealand.
2Agricultural Research and Rural Extension of Santa Catarina, Santa Catarina, Brazil.
3Federal Institute of Education, Science and Technology of Santa Catarina, Lages, Santa Catarina, Brazil.
Abstract
Rooting tissue-cultured shoots and acclimatizing the rooted plantlets are key final steps in successful micropropagation protocols. We assessed the effect of indole-3-acetic acid (IAA) from synthetic and biological sources for effects on in vitro rhizogenesis and acclimatization stages in micropropagated ‘Marubakaido’ (Malus prunifolia) apple rootstock. Shoots of 3 cm length were transferred to rooting medium, composed of Murashige and Skoog (MS) medium supplemented with up to 2 mg L-1 AIA and grown for 60 days in a controlled environment before assessment. Root initiation rate, callus formation, dry-root biomass, dry-shoot biomass, root length, root volume and diameter were evaluated. After the acclimatization stage, survival rate was determined. The root initiation rate of micropropagated ‘Marubakaido’ apple rootstock was higher in culture media supplemented with IAA, independent of the source. Callus formation at the base of shoots was higher according to increases of synthetic IAA concentration. In contrast, there was no callus formation from shoots cultured on MS medium supplemented with bacterial. ‘Marubakaido’ shoots rooted in vitro were successfully acclimatized. Survival of the plantlets during acclimatization was affected by both IAA concentration and source. Plant survival rate at the acclimatization stage decreased from 96% to 66% as the concentration of synthetic IAA increased. ‘Marubakaido’ inoculated in MS medium supplemented with bacterial IAA had thinner roots without callus formation, resulting in higher survival (up to 98 %) during acclimatization. Strain N39 was particularly effective in inducing in vitro rooting and acclimatization of ‘Marubakaido’ apple rootstock, resulting in high-quality rooted plantlets.
Highlighted Conclusion
1. Supplementation with indole-3-acetic acid (IAA) produced by rhizobacteria during in vitro rooting of apple rootstock increased survival rates at the acclimatization stage, and may be an efficient and sustainable alternative to synthetic IAA within micropropagation protocols.
Communications in Plant Sciences | 2022 | vol.12 | p.016-023
DOI: 10.26814/cps2022003 | Article code: cps2022003
Keywords: Malus prunifolia, Auxins, Micropropagation, Plant growth-promoting rhizobacteria
Correspondence to: Murilo Dalla Costa <murilodc@epagri.sc.gov.br>
Submission on September 15, 2021 | First Publication on February 17, 2022 | Open Access
Authors declared no conflict of interest
Article licensed under a Creative Commons Attribution-NonCommercial 4.0 International
Heritability estimates and genetic distance of s1 progenies from landrace maize populations
INTRODUCTION
Apples (Malus spp.) are among the most important fruit crops worldwide. Globally, apple production reached about 87 Mt in 2019, of which 1.4% was produced in Brazilian orchards (Faostat 2019). Apple producing areas in Brazil are concentrated in the two southernmost states, Santa Catarina and Rio Grande do Sul, which together account for 97.3% of national production (Epagri/Cepa 2019). The success of apple production in Brazil is underpinned by the apple breeding program initiated in the early 1970s (Denardi et al. 2019, Petri et al. 2011). New apple cultivars well adapted to the southern Brazilian climate and resistant to the main apple diseases were imported. In addition, quarantine procedures required that imported apple cultivars be either virus free or cleaned up prior to entry into the country. This increased the productivity and fruit quality of Brazilian apple orchards (Denardi et al. 2019, Petri et al. 2011).
The use of rootstocks to grow apple trees is common practice: initially this was related to difficulties of scion cultivar propagation by cuttings, but nowadays rootstocks are well known for their effects on the growth, yield, vigor, precocity and fruit quality of scions (Robinson 2011, De Ollas et al. 2019, Hezema et al. 2021, Rufato et al. 2021, Xu and Ediger 2021). In addition, rootstocks can confer various pest and disease resistances to grafted cultivars (Denardi et al. 2015,2016, Wang et al. 2019). ‘Marubakaido’ (M. prunifolia) apple rootstock is often used for cultivated apples in Brazil, as its root system is very vigorous and adapted to most types of soil, showing resistance to pathogens and is thus a good alternative for replanted soils (Denardi 2006, Denardi et al. 2018). Furthermore, this species is also recommended for many provinces in China (Wang et al. 2019). Propagation using in vitro micropropagation, supplements conventional propagation methods in the production of healthy, disease-free plants and in the rapid multiplication of plant material with desirable traits throughout the year (Dobránszki and da Silva 2010, Bettoni et al. 2016, 2019, Souza et al. 2020). In addition, in vitro techniques provide a complementary strategy to conserve germplasm collections for future requirements of breeding programs (Bhatti and Jha 2010, Feng et al. 2013, Li et al. 2015, Wang et al. 2018, Bettoni et al. 2021a,b).
In vitro propagation of apple can be divided into four stages: establishment; shoot multiplication; rooting of micro-shoots; and acclimatization (transfer from in vitro to ex vitro conditions) of in vitro plantlets (Da Silva et al. 2019, Zhang et al. 2020). Multiplication of shoots is achieved by promoting the growth of axillary shoots or induction of adventitious shoots in cytokinin-supplemented culture media (Dobránszki and da Silva 2010, Magyar- Tábori et al. 2010, Saeiahagh et al. 2019). It can be difficult to induce roots on shoots originating from this multiplication stage under normal conditions. Therefore, in most plants, to improve in vitro rooting, shoots are rooted on culture media enriched with auxins such as indole-3-acetic acid (IAA), indole-3-butyric acid (IBA) and 1-naphthaleneacetic acid (NAA) (Centellas et al. 1999, Radmann et al. 2002, Jardim et al. 2010, Magyar- Tábori et al. 2011, Lizárraga et al. 2017, Modgil and Thakur 2017, Bettoni et al. 2018).
Studies have demonstrated the potential of rhizobacteria to produce IAA and their associated beneficial effects when introduced into in vitro micropropagation protocols at the rooting stage (PeyvandI et al. 2010, Muniz et al. 2013, Dalla Costa et al. 2018). Plant growth promoting bacteria (PGPB) have been shown to be capable of colonizing the rhizosphere of plant roots and producing growth regulating compounds such as siderophores, hydrocyanic acid (HCN) and antibiotics; these directly and indirectly influence the growth of the hosts (Ahmad et al. 2005, Mohite 2013, Souza et al. 2017). The use of PGPB in plant growth experiments has therefore increased substantially in the last few decades (Kudoyarova et al. 2019). The use of PGPB to promote plant growth under drought conditions, in high salinity, in soils contaminated with toxic metals, and agricultural systems with reduced fertilizer and pesticide application, without compromising crop yield and environmental benefits, is attracting increased attention (Belimov et al. 2009, Gerhardt et al. 2009, Vacheron et al. 2013, Passos et al. 2014, Kundan et al. 2015, Pii et al. 2015, Habib et al. 2016, Parray et al. 2016, Gouda et al. 2018, Arifin et al. 2021). However, the use of PGPB directly in in vitro cultures is still in its infancy: more commonly IAA is produced from PGPB and then applied discretely. Therefore, the aim of this study was to assess the use of IAA from synthetic and biological sources in the in vitro rhizogenesis and acclimatization stages in micropropagated ‘Marubakaido’ apple rootstock.
MATERIAL AND METHODS
Plant material and stock cultures. In vitro shoots of ‘Marubakaido’ apple rootstock were subcultured in MS medium (Murashige and Skoog 1962) supplemented with 40 g L-1 sucrose, 1 mg L-1 6-benzylaminopurine (BAP) and 7 g L-1 of agar. The medium pH was adjusted to 5.8 prior to autoclaving at 121 °C for 15 min. Cultures were maintained in a growth chamber at 25°C ± 2 °C, under a photoperiod of 16 h light day-1 with a light intensity of 50 μmol m-2 s-1.
Strain selection and IAA production. The bacterial strain identified as N39 was isolated from rhizosphere soil of Axonopus catharinensis Valls. This strain was selected based on its IAA production potential in a preliminary research at Epagri’s Laboratory of Biotechnology, in Lages, Santa Catarina State, Brazil (Souza et al. 2017). The strain N39 is registered within the Brazilian national genetic heritage collection in the Sistema Nacional de Gestão do Patrimônio Genético e do Conhecimento Tradicional Associado (SISGEN) under registration number AB5910A.
Production of IAA by isolate N39 was analyzed using a colorimetric method developed by Asghar et al. (2002). Strain N39 was grown on yeast-mannitol-agar (YMA) medium supplemented with 250 mg L-1 tryptophan and incubated at 25 °C for 72 h. Then, 2 mL aliquots of bacterial suspension were centrifuged at 5,000 rpm for 5 min at 4 °C. Supernatant was reserved and 100 μL was transferred to a microplate well with 100 μL of Salkowski’s solution (0.5 M FeCl3 in 35% HClO4 solution). The microplate containing the mixture was incubated at room temperature, for 30 min, in the dark, and which the absorbance was measured at 530 nm in a spectrophotometer. The isolate N39 produced 30.2 μg mL-1 IAA. Then, aliquots corresponding to IAA concentrations evaluated were added to the culture medium.
Experimental design. The experimental design was a randomized block design in a 2 x 5 factorial arrangement, consisting of two sources of IAA (synthetic and bacterial) and five IAA concentrations (0, 0.5, 1.0, 1.5 or 2 mg L-1), with three replicates of 20 shoots per replicate. A commercial product (Sigma, I2886) was used as the synthetic source and strain N39 as the bacterial source.
To assess survival rate at the acclimatization stage, all cultures were grown for 60 days on in vitro rooting medium, after which twenty of the rooted plantlets from each treatment were randomly selected and potted in commercial substrate.
In vitro rooting. ‘Marubakaido’ shoots 3 cm in length containing three leaves were collected from 45-day-old in vitro stock cultures, and placed in test tubes with MS medium supplemented with 30 g L-1 of sucrose, IAA from synthetic or biological source (according to treatments), 7 g L-1 agar, with the medium pH adjusted to 5.8 prior to autoclaving at 121 °C for 15 min. The cultures were grown under the same conditions as the stock cultures and after 60 days, in vitro rooted cultures were acclimatized.
Acclimatization. The acclimatization was performed according to Bettoni et al. (2019). In vitro rooted cultures were pruned by preserving two to three basal leaves and 2 cm root length. Plantlets were then transferred to 100 mL honeycomb trays containing commercial substrate Tecnomax® (Tecnomax, Vargem Bonita, Santa Catarina, Brazil) and sand (3:2 v/v). The substrate mixture had been heat-sterilized at 121 °C for 1 h. Each tray was placed in a plastic basin covered with glass to maintain high relative humidity. The plantlets were maintained in a growth room under the same conditions as the stock cultures. The glass cover was gradually removed once new leaves appeared. Plantlets were grown for 45 days following transfer to open greenhouse bench before the plant survival rate was evaluated.
Data collection and analysis. Rooting and callus formation were determined in in vitro cultures after 60 days of growth on rooting medium, while the plantlet survival rates of the micropropagated ‘Marubakaido’ were determined after potted plants had spent 45 days at the acclimatization stage.
For in vitro rooting evaluation, the rooting rates (%) and callus formation (%) were assessed before shoots and roots were separated: roots then were washed in tap water and stored in 50% ethanol in preparation for taking images that were analyzed for total root length, root surface area, average root diameter, root volume, and number of forks and tips using WinRhizo Pro scanning software (Regent Instruments, Quebec, Canada) system with the Epson Expression 10000 XL scanner. The dry biomass was determined in shoots and roots after oven-drying at 65 – 70 °C for 72 h.
Data were submitted to a regression analysis and analysis of variance (ANOVA) followed by Tukey post-hoc test (p ≤ 0.05). Statistical procedures were performed using the R software (R Development Core Team 2014).
RESULTS AND DISCUSSION
The rooting rate of micropropagated ‘Marubakaido’ apple rootstock was higher in culture media supplemented with IAA, independent of the source (Figure 1A). The highest predicted rooting rate using synthetic IAA, according to a polynomial regression curve, was at 1.3 mg L-1 of IAA (Figure 1A). However, for bacterial IAA treatments, there was a positive linear effect on rooting rates according to the regression curve (Figure 1A). Similar effects on the in vitro rooting of ‘Marubakaido’ apple rootstock were reported by Muniz et al. (2013) who applied a sterilized culture broth from rhizobia isolated from Adesmia latifolia root nodules.
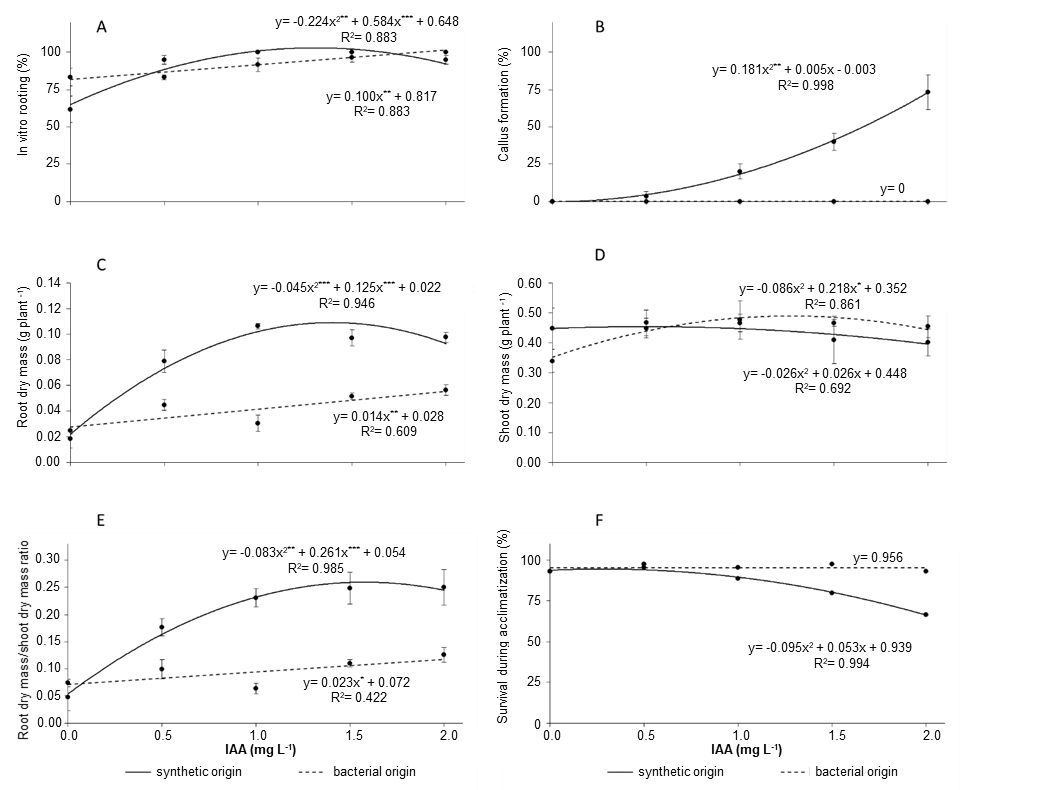
Figure 1. Effects of culture media supplemented with indole-3-acetic acid (IAA) from synthetic or bacterial sources on the root initiation rate (A), callus formation (B), root dry mass (C), shoot dry mass (D), root dry mass/shoot dry mass ratio (E) and survival rate (F) of in vitro apple (Malus prunifolia ‘Marubakaido’). Asterisks represent significant differences as calculated by ANOVA (*p<0.05, **p<0.01, ***p<0.001).
Callus formation at the base of shoots increased with increasing synthetic IAA concentration; in contrast, there was no callus formation in shoots cultured on MS medium supplemented with bacterial IAA (Figure 1B and Figure 2). Callus is an irregular structure formed during the rooting process. Although calli are not root primordia, they have gene expression profiles similar to that of root meristems, suggesting there is some overlap in the genetic control of callus formation and root primordium induction (Ikeuchi et al. 2013, Lee et al. 2018, Shin and Seo 2018). In addition to stimulating root induction, auxin application often leads to callus formation and expression of genes that are not necessarily related to root initiation (Welander et al. 2014). Callus has a negative effect on shoot growth and root formation, mainly because its undifferentiated structure impairs the vascular connection between roots and shoots, preventing the absorption of water and nutrients (Radmann et al. 2002, Arab et al. 2018). Therefore, a rooting method leading to no or little callus formation is desirable (Welander et al. 2014, Srikanth et al. 2016).
The addition of synthetic IAA increased root dry mass (RDM) (Figure 1C) compared to treatments with bacterial IAA application (p <0.001). However, IAA source had no effect on shoot dry mass (SDM) (p = 0.3326; Figure 1D). Thus, the RDM/SDM ratio within synthetic IAA treatments was consistently and significantly higher for all IAA concentrations evaluated, compared with bacterial IAA application (Figure 1E).

Figure 2. In vitro rooted plants of apple (Malus prunifolia ‘Marubakaido’) on Murashige and Skoog (MS) medium supplemented with 0.5 (left) and 2.0 mg L-1 (right) indole-3-acetic acid (IAA) from synthetic (A) and bacterial (B) sources. White arrow indicates callus formation. Scale bars: A= 1 cm, B= 0.8 cm.
‘Marubakaido’ shoots rooted in vitro were able to be successfully acclimatized. Survival of plantlets during the acclimatization was affected by both IAA concentration and source (Figure 1F). Plant survival rate at the acclimatization stage decreased from 96% to 66% as the concentration of synthetic IAA increased, i.e., survival decreased as synthetic IAA level increased (Figure 1F). Survival was 98% and 93% in ‘Marubakaido’ plants previously grown in MS medium supplemented with bacterial IAA at 0.5 mg L-1 and 2 mg L-1, respectively (Figure 1F). The success of micropropagation depends on effective acclimatization and the reduction of plant losses at this final stage has a strong economic impact. We observed a relationship between callus formation in vitro and survival at the acclimatization stage, wherein callus formation had a negative impact on acclimatization. According to our results, higher concentrations of synthetic IAA during the rooting stage enhanced callus formation, which subsequently decreased the survival of plants during acclimatization (Figures 1B and 1F). The results obtained here are similar to those reported by Arab et al. (2018) on Prunus in vitro rooting using synthetic IBA. IBA has been suggested as a suitable hormone for in vitro rooting of Prunus rootstock, however, higher concentrations of IBA during the rooting stage enhanced callus formation and increased plant losses at the acclimatization stage (Arab et al. 2018).
The root morphology and architecture results followed that the root dry mass responses, with highest predicted values in the presence of synthetic IAA being 1.2 and 1.3 mg L-1, respectively (Figure 3). ‘Marubakaido’ plants grown in MS medium supplemented with 0.5, 1.0, and 1.5 mg L-1 synthetic IAA had better root development (i.e., increased total root length, surface area, root average diameter, volume occupied in soil, number of forks and number of tips) compared with the corresponding bacterial IAA concentrations (Figure 3). Interestingly, the thick roots produced by ‘Marubakaido’ plantlets rooted in the presence of synthetic IAA were more fragile during acclimatization. While the presence of a root is positive, the presence of fibrous and thick roots lacking root hairs, sometimes associated with callus formation, may be detrimental for the acclimatization of micropropagated plants (Kataoka 1994, Karhu 1997, Radmann et al. 2002,2014, Wang et al. 2020). In contrast, shoots of ‘Marubakaido’ cultured in MS medium supplemented with bacterial IAA had thinner roots without callus formation, resulting in a higher survival rate during acclimatization (Figure 1F, Figure 2).
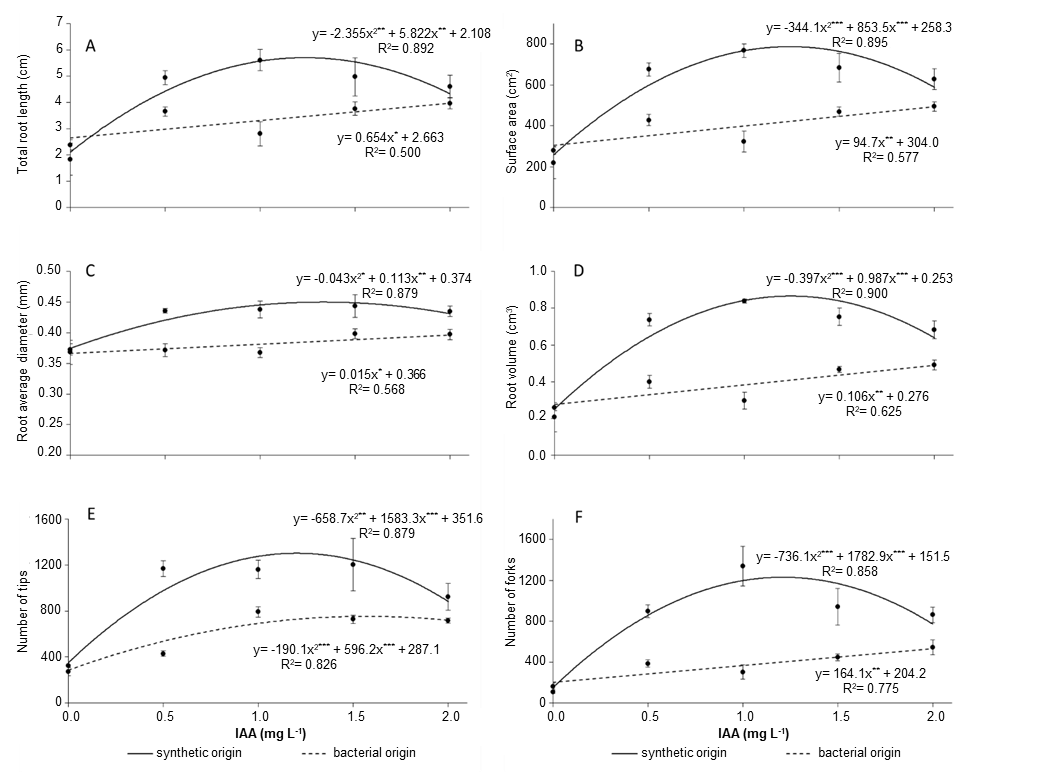
Figure 3. Effects of culture media supplemented with indole-3-acetic acid (IAA) from synthetic or bacterial sources on the total root length (A), surface area (B), root average diameter (C), root volume (D), number of tips (E) and number of forks (F) of in vitro propagated apple (Malus prunifolia ‘Marubakaido’). Asterisks represent significant differences as calculated by ANOVA (*p<0.05, **p<0.01, ***p<0.001).
Studies have shown that a wide range of PGPB are capable of promoting plant growth and are involved in the production of IAA (Asghar et al. 2002, Mohite 2013, Muniz et al. 2013, Wang et al. 2020, Alemneh et al. 2021). Furthermore, many of these that produce IAA have been shown to be effective in protecting plants against abiotic and biotic stresses (Beneduzi et al. 2012, Bhattacharyya and Jha 2012, Reetha et al. 2014, Arifin et al. 2021). The strain N39, isolated from the rhizosphere soil of Axonopus catharinensis Valls, produced 30.2 μg mL-1 IAA and its incorporation (sterilized culture broth) in MS medium to produce an IAA concentration between 0.5 and 2.0 mg L-1 was effective in improving both in vitro rooting and acclimatization of ‘Marubakaido’ apple rootstock, resulting in high-quality rooted plantlets and a high survival rate during acclimatization. Further studies may identify other compounds generated in the bacterial broth and also to explore the use of bacterial IAA in in vitro and ex vitro rooting in other plant species as well as acclimatization of micropropagated shoots in addition to ‘Marubakaido’ apple rootstock.
Acknowledgements
The authors are grateful to the Federal Institute of Santa Catarina (IFSC/Brazil) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq/Brazil) project MDA/CNPq 2014-8 (Process CNPq 472977/2014-8), for the research scholarship granted to Juliana Aparecida Souza and for financial support; to the Agricultural Research and Rural Extension of Santa Catarina (EPAGRI) for providing facilities and technical support for the development of this research. JCB thanks Ranjith Pathirana (Waite Research Precinct, Australia) and Sathiyamoorthy Meiyalaghan (Lincoln Research Centre, New Zealand) from Plant & Food Research for providing internal review of the manuscript.
References
- Ahmad F et al. 2005. Indole acetic acid production by the isolates of Azotobacter and Fluorescent Pseudomonas in the presence an absence of tryptophan. Turkish Journal of Biology 29:29–34.
- Alemneh et al. 2021. Ability to produce indole acetic acid is associated with improved phosphate solubilising activity of rhizobacteria. Archives of Microbiology 203:3825–3837.
- Arab MM et al. 2018. Modelling and optimizing a new culture medium for in vitro rooting of G×N15 Prunus rootstock using artificial neural network-genetic algorithm. Scientific Reports 8:9977.
- Arifin MS et al. 2021. Salt tolerant rhizobacteria from coastal region of Bangladesh portrayed the potential for plant growth promotion. Journal of Applied Life Sciences International 24:58–70.
- Asghar H et al. 2002. Relationship between in vitro production of auxins by rhizobacteria and their growth-promoting activities in Brassica juncea L. Biology and Fertility of Soils 35:231–237.
- Belimov A et al. 2009. Rhizosphere bacteria containing 1-aminocyclopropane-1-carboxylate deaminase increase yield of plants grown in drying soil via both local and systemic hormone signaling. New Phytologist 181:413–423.
- Beneduzi A et al. 2012. Plant growth-promoting rhizobacteria (PGPR): Their potential as antagonists and biocontrol agents. Genetics and Molecular Biology 35:1044–1051.
- Bettoni JCB et al. 2016. Cryotherapy: a new technique to obtain grapevine plants free of viruses. Revista Brasileira de Fruticultura 38:1–13.
- Bettoni JC et al. 2018. Cryotherapy by encapsulation-dehydration is effective for in vitro eradication of latent viruses from ‘Marubakaido’ apple rootstock. Journal of Biotechnology 269:1–7.
- Bettoni JC et al. 2019. Eradication of latent viruses from apple cultivar ‘Monalisa’ shoot tips using droplet-vitrification cryotherapy. Scientia Horticulturae 250:12–18.
- Bettoni JC et al. 2021a. Challenges in implementing plant shoot tip cryopreservation technologies. Plant Cell, Tissue and Organ Culture 144:21–34.
- Bettoni JC et al. 2021b. Grapevine shoot tip cryopreservation and cryotherapy: secure storage of disease-free plants. Plants, 10:2190.
- Bhattacharyya PN and Jha DK. 2012. Plant growth-promoting rhizobacteria (PGPR): emergence in agriculture. World Journal of Microbiology and Biotechnology 28:1327–1350.
- Bhatti S and Jha G. 2010. Current trends and future prospects of biotechnological interventions through tissue culture in Apple. Plant Cell Reports 29:1215–1225.
- Centellas AQ et al. 1999. Efeito de auxinas sintéticas no enraizamento in vitro de macieira. Pesquisa Agropecuária Brasileira 34:181–186.
- Da Silva, JAT. et al. 2019. In vitro tissue culture of apple and other Malus species: recent advances and application. Planta 249: 975–1006.
- Dalla Costa M et al. 2018. Indução de enraizamento in vitro e micorrização ex vitro de culturas de Muscadinia rotundifolia. In: I Simpósio de Integração da Pós-Graduação: Ciência, Tecnologia e Inovação. Lages (SC) CAV/UDESC. Available at https://www.even3.com.br/anais/siga/80085-inducao-de-enraizamento-in-vitro-e-micorrizacao-ex-vitro-de-culturas-de-muscadinia-rotundifolia/. Accessed on August 16, 2021
- De Ollas C et al. 2019. Facing climate change: biotechnology of iconic mediterranean woody crops. Frontiers in Plant Science 10:1–23.
- Denardi F. 2006. Porta enxertos. In: Epagri. A cultura da macieira. Editora Pallotti, Florianópolis. pp.169–227.
- Denardi F et al. 2015. Porta-enxertos de macieira: passado, presente e futuro. Agropecuária Catarinense 28:89–95.
- Denardi F et al. 2016. Performance of new apple rootstocks for Gala variety in Southern Brazil. Crop Beeding and Applied Biotechnology 16:147–152.
- Denardi F et al. 2018. Yield performance of apple rootstocks of the Geneva series on replanting soil. Pesquisa Agropecuaria Brasileira 53:924–933.
- Denardi F et al. 2019. A brief history of the forty-five years of the Epagri apple breeding program in Brazil. Crop Breeding and Applied Biotechnology 19:347–355.
- Dobránski J and Da Silva JAT. 2010. Micropropagation of Apple – A review. Biotechnology Advances 28:462–488.
- Epagri/Cepa. Síntese Anual da Agricultura de Santa Catarina 2018 – 2019. Available at https://docweb.epagri.sc.gov.br/website_cepa/publicacoes/Sintese_2019_20.pdf. Accessed on August 16, 2021.
- Feng CH et al. 2013. Duration of sucrose preculture is critical for shoot regrowth of in vitro-grown apple shoot-tips cryopreserved by encapsulation-dehydration. Plant Cell, Tissue and Organ Culture 112:369–378.
- Faostat – Food and Agriculture Organization of the United Nations / FAOSTAT: global production statistics. Available at http://www.fao.org/faostat/en/#data. Accessed on August 16, 2021.
- Gerhardt KE et al. 2009. Phytoremediation and rhizoremediation of organic soil contaminants: Potential and challenges. Plant Science 176:20–30.
- Gouda S et al. 2018. Revitalization of plant growth promoting rhizobacteria for sustainable development in agriculture. Microbiological Research 206:131–140.
- Habib SH et al. 2016. Plant growth-promoting rhizobacteria enhance salinity stress tolerance in okra through ROS-scavenging enzymes. BioMed Research International 2016:1–10.
- Hezema YS et al. 2021. Physiological and molecular responses of six apple rootstocks to osmotic stress. International Journal of Molecular Sciences 22:8263.
- Ikeuchi M et al. 2013. Plant Callus: mechanisms of induction and repression. The Plant Cell 25:3159–3173.
- Jardim LS et al. 2010. Efeito de diferentes reguladores de crescimento na regeneração in vitro de pau-rosa (Aniba rosaedora Ducke). Acta Amazonica 40:275–280.
- Karhu ST. 1997. Rooting of blue honeysuckle microshoots. Plant Cell, Tissue and Organ Culture 48:153–159.
- Kataoka I. 1994. Influence of rooting substrates on the morphology of papaya root formed in vitro. Journal of Tropical Agriculture 38:251–257.
- Kudoyarova G et al. 2019. Phytohormone mediation of interactions between plants and non-symbiotic growth promoting bacteria under edaphic stresses. Frontiers in Plant Science 10:1368.
- Kundan R et al. 2015. Plant growth promoting rhizobacteria: mechanism and current prospective. Journal of Agricultural Science and Food Research 6:1–9.
- Lee K et al. 2018. JMJ30-mediated demethylation of H3K9me3 drives tissue identity changes to promote callus formation in Arabidopsis. The Plant Journal 95:961–975.
- Li BQ et al. 2015. Shoot tip culture and cryopreservation for eradication of Apple stem pitting virus (ASPV) and Apple stem grooving virus (ASGV) from apple rootstocks ‘M9’ and ‘M26’. Annals of Applied Biology 168:1–9.
- Lizárraga A et al. 2017. In vitro propagation and recovery of eight apple and two pear cultivars held in a germplasm bank. American Journal of Plant Sciences 8:2238–2254.
- Magyar-Tábori K et al. 2010. The role of cytokinins in shoot organogenesis in apple. Plant Cell, Tissue and Organ Culture 101:251–267.
- Magyar-Tábori K et al. 2011. Effect of cytokinin content of the regeneration media on in vitro rooting ability of adventitious apple shoots. Scientia Horticulturae 129:910–913.
- Modgil M and Thakur M. 2017. In vitro culture of clonal rootstocks of apple for their commercial exploitation. Acta Horticulturae 1155:331–335
- Mohite B. 2013. Isolation and characterization of indole acetic acid (IAA) producing bacteria from rhizospheric soil and its effect on plant growth. Journal of soil science and Plant Nutrition 13:638–649.
- Muniz AW et al. 2013. Rooting and acclimatization of micropropagated Marubakaido apple rootstock using Adesmia latifolia rhizobia. Springer Plus 2:1–6.
- Murashige T, Skoog F. 1962. A revised medium for rapid growth and bioassay with tobacco tissue cultures. Physiology plant 15:473–497.
- Parray JA et al. 2016. Current perspectives on plant growth-promoting rhizobacteria. Journal of Plant Growth Regulation 35:877–902.
- Passos JFM et al. 2014. Cultivate bacteria isolated from apple trees cultivated under different crop systems: Diversity and antagonistic activity against Colletotrichum gloesporioides. Genetics and Molecular Biology 37:560–572.
- Petri JL et al. 2011. Avanços na cultura da macieira no Brasil. Revista Brasileira de Fruticultura 33:48–56.
- Peyvandi M et al. 2010. Pseudomonas fluorescent and its ability to promote root formation of olive microshoots. International Journal of Plant Production 4:63–66.
- Pii Y et al. 2015. Microbial interactions in the rhizosphere: beneficial influences of plant growth-promoting rhizobacteria on nutrient acquisition process. A review. Biology and Fertility of Soils 51:403–415.
- R Development Core Team. 2014. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. https://www.r-project.org/
- Radmann EB et al. 2002. Efeito de auxinas e condições de cultivo no enraizamento in vitro de porta-enxertos de macieira M-9. Revista Brasileira de Fruticultura 24:624–628.
- Radmann EB et al. 2014. Enraizamento in vitro e aclimatização do porta-enxerto de ameixeira ‘MR. S.2/5’. Plant Cell Culture & Micropropagation 19:21–31.
- Reetha S et al. 2014. Isolation of indole acetic acid (IAA) producing rhizobacteria of Pseudomonas fluorescens and Bacillus subtilis and enhance growth of onion (Allim cepa L). International Journal of Current Microbiology and Applied Sciences 3:568–574.
- Robinson T. 2011. Advances in apple culture worldwide. Revista Brasileira de Fruticultura 33:37–47.
- Rufato L et al. 2021. Geneva® series rootstocks for apple trees under extreme replanting conditions in southern Brazil. Frontiers in Plant Science 12:1–13.
- Saeiahagh H et al. 2019. Effect of cytokinins and sucrose concentration on the efficiency of micropropagation of ‘Zes006’ Actinidia chinensis var. chinensis, a red-fleshed kiwifruit cultivar. Plant Cell, Tissue and Organ Culture 138:1–10.
- Shin J and Seo PJ. 2018. Varying auxin levels induce distinct pluripotent states in callus cells. Frontiers in Plant Science 9:1653.
- Souza JA et al. 2017. Diversidade e potencial biotecnológico da comunidade bacteriana diazotrófica de missioneira-gigante (Axonopus catharinensis). In: IV Simpósio Internacional Ciência, Saúde e Território, 2017, Lages, Brazil. Available at http://www.simposioppgas.com.br/downloads/ANAIS_SIMPOSIO2017.pdf. Accessed on August 17, 2021.
- Souza JA et al. 2020. Droplet-vitrification cryoterapy for eradication of apple stem grooving virus and apple stem pitting virus form “Marubakaido” apple rootstock. Tropical Plant Pathology 45:148–152.
- Srikanth S et al. 2016. An efficient method for adventitious root induction from stem segments of Brassica species. Frontiers in Plant Science 7:943.
- Vacheron J et al. 2013. Plant growth-promoting rhizobacteria and root system functioning. Frontiers in Plant Science 4:1–19.
- Wang MR et al. 2018. Cryobiotechnology of apple (Malus spp.): development, progress and future prospects. Plant Cell Reports 37:689–709.
- Wang Y et al. 2019. Progress of apple rootstock breeding and its use. Horticultural Plant Journal 5:181–191.
- Wang F et al. 2020. In vitro regeneration, ex vitro rooting and foliar stoma studies of Pseudostellaria heterophylla (Miq.) Pax. Agronomy 10:949.
- Welander M et al. 2014. Origin, timing, and gene expression profile of adventitious rooting in Arabidopsis hypocotyls and stem. American Journal of Botany 101:255–266.
- Xu H and Ediger D. 2021. Rootstocks with different vigor influenced scion-water relations and stress responses in AmbrosiaTM apple trees (Malus Domestica var. Ambrosia). Plants 10:614.
- Zhang Y et al. 2020. An efficient in vitro regeneration system from different wild apple (Malus sieversii) explants. Plant Methods 16:56.
